
The Field
The work done under the heading of Materials Science Engineering
has an unprecedented impact on our quality of life. Although the field
deals with materials, it encompasses an incredible diversity of topics and
problems constituting the four elements of the field -- processing,
structure, properties, and performance.
 Materials Materials
History is measured by innovations in materials. Developments in metals
like iron and bronze enabled advances in civilization thousands of years
ago, a synergy which continues today in the fiber optics that have created
the internet and in the development of biomaterials that mimic
living tissue. As you explore the field it may be useful to become
familiar with some generic categories of materials.
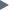 Metals Metals
Metals are materials that are normally combinations of "metallic
elements." These elements, when combined, usually have electrons that are
non-localized and as a consequence have generic types of properties.
Metals usually are good conductors of heat and electricity. They are also
quite strong but deformable and tend to have a lustrous look when
polished.
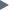 Ceramics Ceramics
Ceramics are generally compounds between metallic and nonmetallic elements
and include such compounds as oxides, nitrides, and carbides. Typically
they are insulating and resistant to high temperatures and harsh
environments.
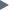 Plastics Plastics
Plastics, also known as polymers, are generally organic compounds based
upon carbon and hydrogen. They are very large molecular structures.
Usually they are low density and are not stable at high temperatures.
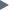 Semiconductors Semiconductors
Semiconductors have electrical properties intermediate between metallic
conductors and ceramic insulators. Electrical properties are strongly
dependent upon small amounts of impurities.
 Composites Composites
Composites consist of
more than one material type. Fiberglass, a combination of glass and a
polymer, is an example. Concrete and plywood are other familiar
composites. Many new combinations include ceramic fibers in metal or
polymer matrix.
 Processing Processing
Processing refers to the way in which a material is achieved. Advances in
technology have made it possible to create a material atomic layer by
atomic layer. There are four general categories which may be useful to
know: solidification processing, powder processing, deposition processing,
and deformation processing.
 Solidification Processing Solidification Processing
Most metals are formed
by creating an alloy in the molten state, where it is relatively easy to
mix the components. This process is also utilized for glasses and some
polymers. Once the proper temperature and composition have been achieved,
the melt is cast. Castings can be divided into two types, depending on the
subsequent processing steps. The first type is shape casting, which takes
advantage of the fluidity of liquid metal to form complex shapes directly.
Because of the complexity of their part geometries, these castings
generally cannot be worked mechanically to a significant degree. Therefore
any changes in microstructure or properties must either be achieved first
during solidification or through subsequent heat treatments.
 Powder Processing Powder Processing
Powder processing
involves consolidation, or packing, of particulate to form a `green body'.
Densification follows, usually by sintering. There are two basic methods
of consolidating powders: either dry powder can be compacted in a die, a
process known as dry-pressing, or the particles can be suspended in a
liquid and then filtered against the walls of a porous mold in a process
known as slip-casting or filter pressing. Bulk ceramics are usually
processed in powder form since their high melting points and low
formability prohibit other types of processing. Metals and polymers can
also be processed from powders.
 Deposition Processing Deposition Processing
Deposition processing
modifies a surface chemically, usually by depositing a chemical vapor or
ions onto a surface. It is used in semiconductor processing and for
decorative or protective coatings. Vapor source methods require a vacuum
to transport the gaseous source of atoms to the surface for deposition.
Common vapor sources are thermal evaporation (similar to boiling water to
create steam), sputtering (using energetic ions to bombard a source and
create the gas state), or laser light (ablates, or removes, atoms from
surface to create the gaseous state). Other sources use carrier media such
as electrochemical mixtures (ions in a solution transported by an
electrical field to the surface for depositions) or spray coating (ions or
small particles transported by gases, liquids, and/or electrical field).
 Deformation Processing Deformation Processing
One of the most common
processes is the deformation of a solid to create a desired shape. A large
force is generally used to accomplish the deformation, and many techniques
heat the material in order to reduce the force necessary to deform it.
Sometimes a mold is used to define the shape. Forging, an old method that
heated the metal and deformed the metal by hammer blows is still used
today, albeit with multi-ton hammers. Rolling to reduce the thickness of a
plate is another common process. Some glasses when heated can be formed
with tools or molds. Other common methods, like drilling to make holes, or
milling, are machining versions of the deformation process.
 Structure Structure
Structure
refers to the arrangement of a material's components from an atomic to a
macro scale. Understanding the structure of a substance is key to
understanding the state or condition of a material, information which is
then correlated with the processing of the material in tandem with its
properties. Understanding these relationships is an intrinsic part of
materials science engineering, as it allows engineers to manipulate the
properties of a material.
 Properties Properties
Does a material need to be strong and heat-resistant, yet lightweight?
Whether you're talking about a fork or the space shuttle, products have
specific requirements which necessitate the use of materials with unique
properties. Materials engineers must frequently reconcile the desired
properties of a material with its structural state to ensure compatibility
with its selected processing. Typical properties of interest may be
classified into:
- Mechanical Properties:
Tensile strength, fracture toughness, fatigue strength, creep
strength, hardness
- Electrical Properties:
Conductivity or
resistivity, ionic conductivity, semiconductor conductivity
(mobility of holes and electrons)
- Magnetic Properties:
Magnetic susceptibility, Curie Temperature, Neel Temperature,
saturation magnetization
- Optical and Dielectric
Properties:
Polarization, capacitance, permittivity, refractive index,
absorption
- Thermal Properties:
Coefficient of thermal expansion, heat capacity, thermal
conductivity
- Environmental Related
Properties:
Corrosion behavior, wear behavior
 Performance Performance
The evaluation of performance is an integral part of the field. The
analysis of failed products is often used to obtain feedback on processing
and its control as well as to assist in the initial selection of the
material and in the stages of processing. Testing also ensures that the
product meets performance requirements. In many products the control of
its processing is closely associated with some property test and/or a
structural characterization.
Note:
Some resources in this section are provided by The Minerals, Metals &
Materials Society
and the US Department
of Labor, Bureau of Labor Statistics.
|
|